
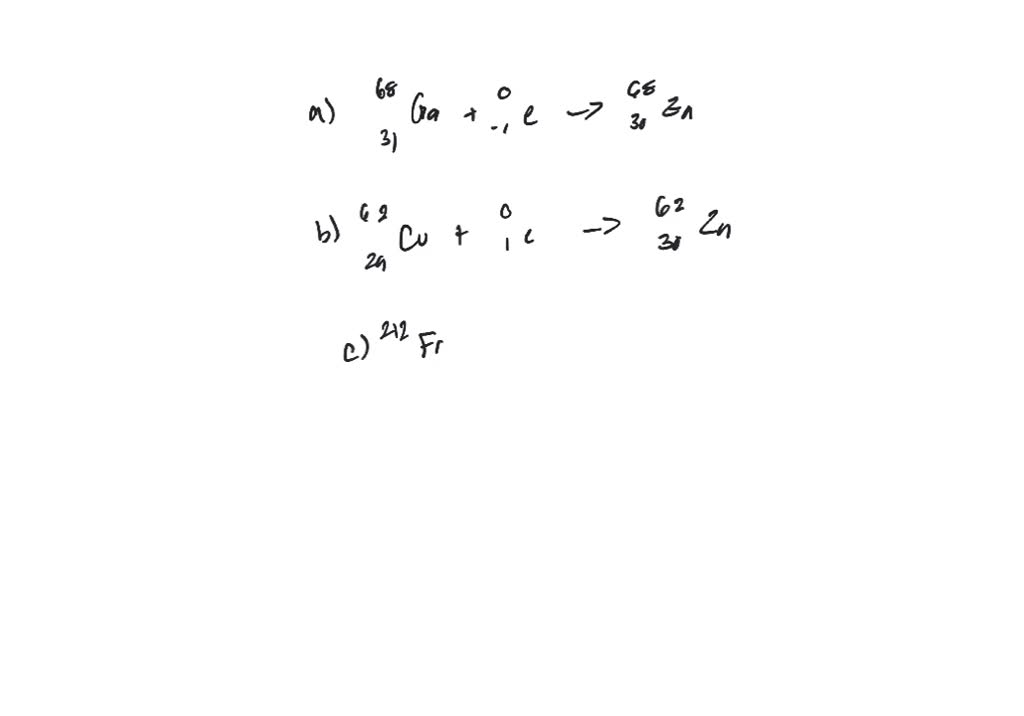
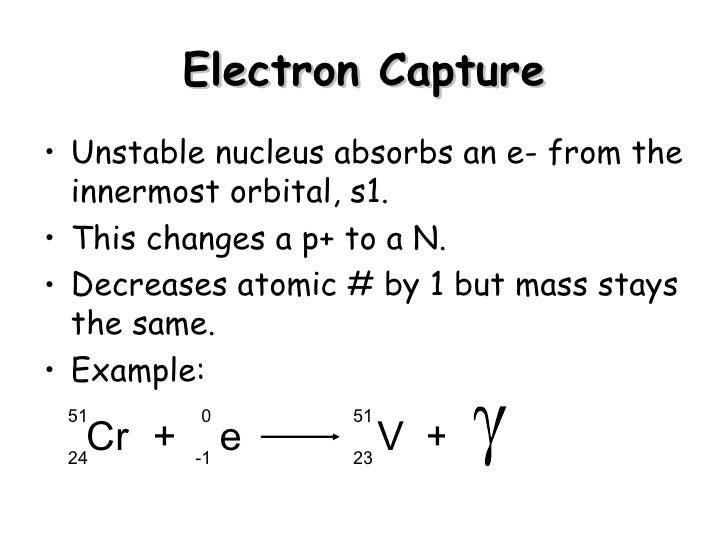
Electron capture is the primary decay mode for isotopes with insufficient energy (Q < 2 x 511 keV) difference between the isotope and its prospective daughter for the nuclide to decay by emitting a positron. This process competes with positive beta decay, which is more common for lighter nuclei. The electron is normally captured from an inner shell of an atom (K-shell). In this process, a parent nucleus may capture one of its orbital electrons and emits a neutrino. In this process, a proton-rich nucleus can also reduce its nuclear charge by one unit by absorbing an atomic electron. Electron capture, also known as inverse beta decay, is sometimes included as a type of beta decay because the basic nuclear process, mediated by the weak interaction, is the same. Electron capture is a process in which a parent nucleus captures one of its orbital electrons and emits a neutrino.


 0 kommentar(er)
0 kommentar(er)
